The mode of action of rilutek is unknown. its pharmacological properties include the following, some of which may be related to its effect: 1) an inhibitory effect on glutamate release, 2). Ilumya™ is a prescription medicine used to treat adults with moderate to severe plaque psoriasis rilutek action who may benefit from taking injections, pills (systemic therapy), or phototherapy (treatment using ultraviolet or uv light). Rilutek 50 mg tablets are white, capsule-shaped, film-coated, and engraved with “rpr 202” on one side. rilutek is supplied in bottles of 60 tablets, ndc 70515-700-60. store at controlled room temperature, 20°c to 25°c (68°f to 77°f), and protect from bright light. patient counseling information.
How goodrx® works.
Riluzole Mnd Australia
Goodrx Medicare
The mode of action of rilutek is unknown. its pharmacological properties include the following, some of which may be related to its effect: 1) an inhibitory effect on glutamate release, 2) inactivation of voltage-dependent sodium channels, and 3) ability to interfere with. We aim to free people from a lifetime of genetic disease. we aim to free people from a lifetime of genetic disease with single-dose gene therapy.
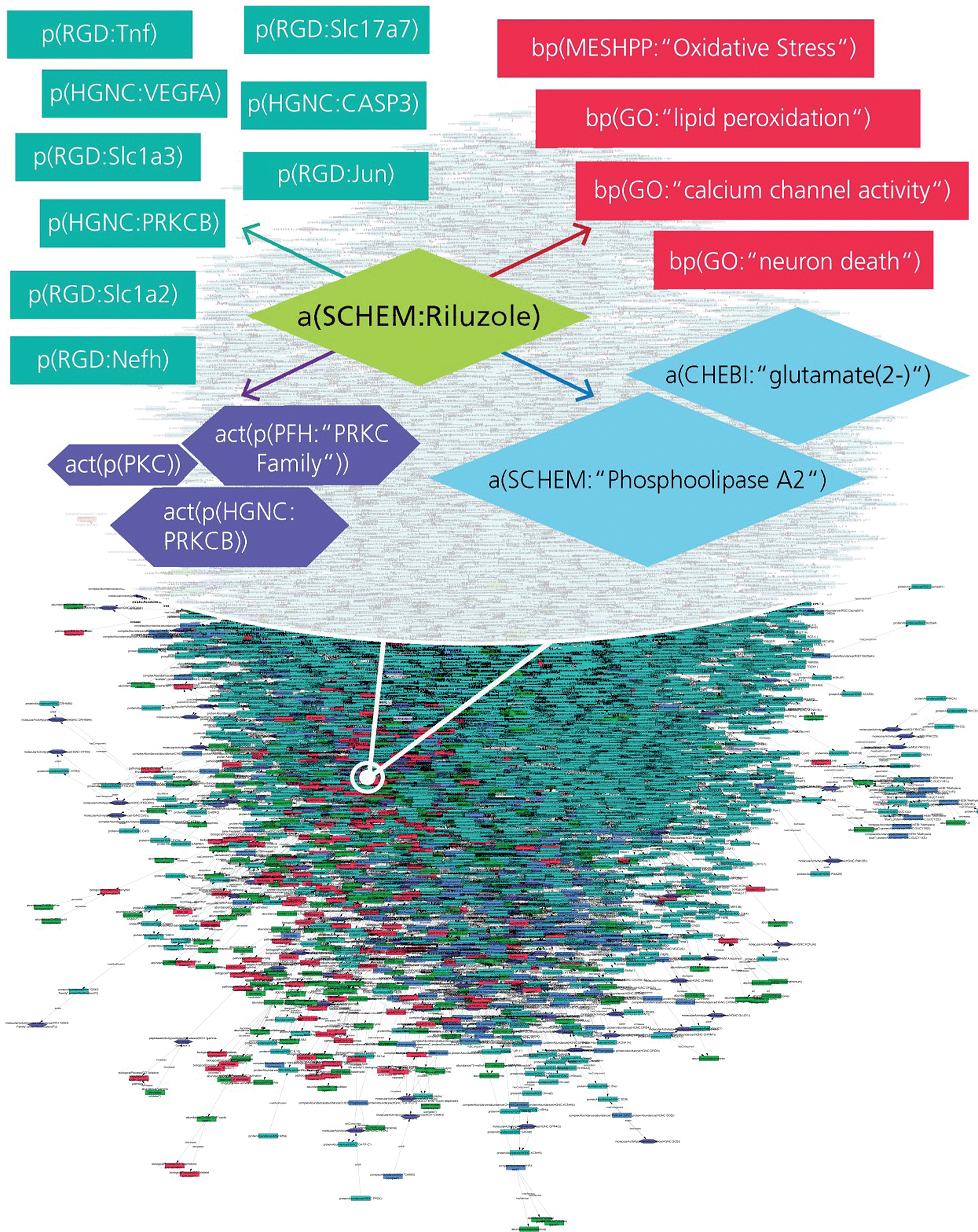
However, it is not a cure for als, and it does not reverse nerve damage or muscle weakness. riluzole is thought to work by protecting the nerves in the brain and . Riluzole acts by binding voltage-gated sodium channels, thereby preventing the propagation of action potentials and eventual axonal release of glutamate [159].
Rilutek Fda Prescribing Information Side Effects And Uses

Riluzole is currently the only drug for the treatment of als, which extends the survival of als patients by an average of 3 months, but its mechanism of action remains unclear with variable patient outcomes. the only other als treatment is rilutek® (riluzole), which was approved in 1995. Call now because you can! parts specialists are ready to look up your parts. Rilutek (riluzole) was the first treatment approved by the u. s. food and drug administration (fda) to treat amyotrophic lateral sclerosis (als). rilutek is an oral formulation that acts to slow the progression of als symptoms and prolong survival. approved in 1995 by the fda, it was originally.
Mechanism of action the mode of action of riluzole is unknown. its pharmacological properties include the following, some of which may be related to its effect: 1) an inhibitory effect on glutamate release (activation of glutamate reuptake), 2) inactivation of voltage-dependent sodium channels, and 3) ability to interfere with intracellular. Cloud-based maintenance management for your business. rated 1 cmms software.
Rilutek is an oral formulation that acts to slow the progression of als symptoms and prolong survival. approved in 1995 by the fda, it was originally developed by the french company rilutek action rhone-poulenc rorer and is now marketed by sanofi. Medscape amyotrophic lateral sclerosis dosing for rilutek, tiglutik (riluzole), frequency-based adverse mechanism of action in patients with als is unknown . The most common adverse reactions in the rilutek group (in at least 5% of patients and more frequently than in the placebo group) were asthenia, nausea, dizziness, decreased lung function, and abdominal pain. The pharmacology and mechanism of action of riluzole the excitotoxic hypothesis of neurodegeneration has stimulated much interest in the possibility of using compounds that will block excitotoxic processes to treat neurologic disorders. riluzole is a neuroprotective drug that blocks glutamatergic neurotransmission in the cns.
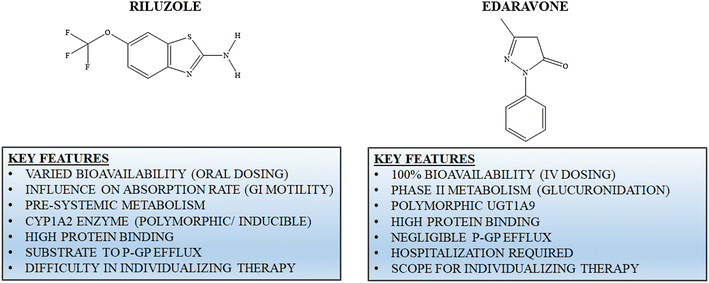
Mechanism of action. riluzole preferentially blocks ttx-sensitive sodium channels, which rilutek action are associated with damaged neurons. riluzole has also been reported to directly inhibit the kainate and nmda receptors. the drug has also been shown to postsynaptically potentiate gaba a receptors via an allosteric binding site. Alt to 5 x uln. readministration of rilutek to patients in this situation cannot be recommended. in cases of rilutek-induced hepatic injury manifested by elevated liver enzymes, the effect of the hepatic injury on rilutek metabolism is unknown (see action and clinical pharmacology, special populations and conditions, hepatic insufficiency).
The excitotoxic hypothesis of neurodegeneration has stimulated much interest in the possibility of using compounds that will block excitotoxic processes to treat neurologic disorders. riluzole is a neuroprotective drug that blocks glutamatergic neurotransmission in the cns. riluzole inhibits the rel. Jun 23, 2020 riluzole is used to treat amyotrophic lateral sclerosis, also known as als or lou gehrig's disease. riluzole is not a cure for als, but it may . Rilutek: protect from light store at controlled room temperature (between 68 and 77 degrees f) tiglutik: discard unused product 15 days after first opening do not freeze protect from light store between 68 to 77 degrees f, excursions permitted 59 to 86 degrees f store upright. Nov 27, 2019 exservan (riluzole oral film) is a medication approved by the u. s. food although the exact mechanism of action of riluzole is not known, it is .
In a second randomized controlled trial, patients were randomized to riluzole 50 mg twice daily or placebo for 3 riluzole modulates the actions of glutamate. Riluzole. the medication, riluzole does not cure motor neurone disease (mnd), but for people with the most common forms of mnd it probably prolongs median . Rilutek 50 mg tablets are white, capsule-shaped, film-coated, and engraved with “rpr 202” on one side. rilutek is supplied in bottles of 60 tablets, ndc 70515-700-60. store at controlled room temperature, 20°c to 25°c (68°f to 77°f), and protect from bright light. Rilutek may harm an unborn baby. use effective birth control to prevent pregnancy, and tell your doctor if you become pregnant. it may not be safe to breastfeed while using rilutek. ask your doctor about any risk. rilutek is not approved for use by anyone younger than 18 years old.
The most common adverse reactions leading to discontinuation in the rilutek group were nausea, abdominal pain, constipation, and elevated alt. there was no difference in rates of adverse reactions leading to discontinuation in females and males. however, the incidence of dizziness was higher in females (11%) than in males (4%). A glutamate antagonist (receptors, glutamate) used as an anticonvulsant (anticonvulsants) and to prolong the survival of patients with amyotrophic lateral sclerosis. riluzole is marketed as rilutek by sanofi. Riluzole is a rilutek action neuroprotective drug that blocks glutamatergic neurotransmission in the cns. riluzole inhibits the release of glutamic acid from cultured neurons, .

0 Response to "Rilutek Action"
Posting Komentar